Aromatics are usually produced from petroleum via chemical conversion processes, which are energy intensive and accompanied by high CO2 emissions.
Using CO2 as a feedstock for the sustainable production of aromatics through catalytic hydrogenation is an economically and environmentally viable approach to address the pressing challenges of energy demand and global warming.
A research team led by Assoc. Prof. SUN Jian, Prof. GE Qingjie and Assoc. Prof. WEI Jian from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences synthesized light aromatics from CO2 hydrogenation by precisely regulating Bronsted acid sites (BAS).
This study was published in Applied Catalysis B: Environmental on October 17.
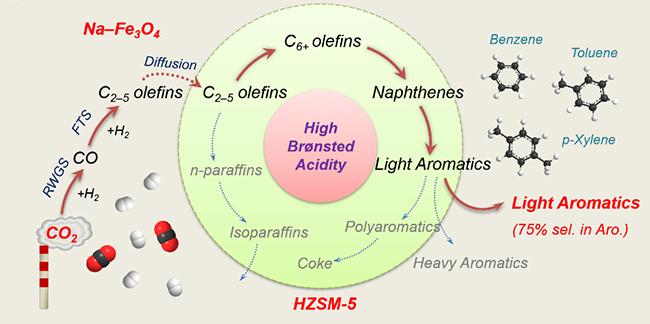
Precisely regulating Bronsted acid sites to promote the synthesis of light aromatics via CO2 hydrogenation (Image by WEI Jian and YAO Ruwei)
The scientists studied a series of composite catalysts comprising Fe-based component and ZSM-5 zeolites with distinct Bronsted acidities to explore the influence of BAS on the light aromatic synthesis and coke formation in CO2 hydrogenation.
They found that BAS of ZSM-5 were the main active sites for aromatization, and the increasing of Bronsted acidity significantly promoted the synthesis of aromatics, especially light aromatics. The further passivation of the external BAS of HZ(25) zeolite by silylation process could inhibit the alkylation of light aromatics and the isomerization of xylene.
The results showed that light aromatics accounted for up to 75% of aromatics, which was the highest value reported in CO2 hydrogenation, and p-xylene could make up as high as 72% of xylene. Moreover, a larger density of BAS, which promoted the formation of highly condensed, carbon-rich, and hard-to-oxidize coke, would accelerate the coke formation, degrade their physico-chemical properties, and shorten the catalyst lifetime.
This work provides a promising strategy to directly synthesize high-valued light aromatics from CO2 and H2, which is importance to cope with energy and environmental problems.
This work was supported by the National Natural Science Foundation of China and the Strategic Priority Research Program of the Chinese Academy of Sciences. (Text and Image by WEI Jian and YAO Ruwei)
Author
WEI Jian and YAO Ruwei
Source
Dalian Institute of Chemical Physics, press release, 2020-12-30.
Supplier
Chinese Academy of Sciences (CAS)
National Natural Science Foundation of China
Share
Renewable Carbon News – Daily Newsletter
Subscribe to our daily email newsletter – the world's leading newsletter on renewable materials and chemicals










