This time we have a bit specific topic in the newsletter – namely effluent salinity. It is an important parameter affecting composition of effluent. There are evolving ways to control salinity level. Vesa Mikkonen will tell some more in the following article. Read and enjoy.
Reducing of effluent salinity at a biorefinery
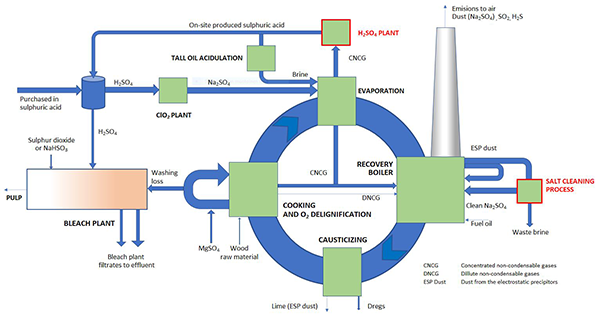
Sulphate discharges have traditionally been not on issue in the case of biorefineries. Times have changed and today sulphate is seen as one emission parameter among the others. Reduction of sulphur emissions to air lead excess sulphur increasingly into the effluent waters. In past days sulphur was balanced through SO2 emissions to air and non-process elements escaped with washing loss and ESP dust from the process.
Sulphur is essential and irreplaceable element in the cooking chemical circle of Kraft pulping process. Sulphur enters the mill mainly as sulfuric acid, which is needed in chlorine dioxide production. In case SW pulp production, acidulation of tall oil the second main route. Small amount of sulphur enters also with MgSO4, which is used as protective chemical in oxygen delignification stage. In past days significant amounts of sulphur entered also with heavy fuel oil but this route has seized as the sulphur content of fuel oil has dropped together with lower demand for additional fuels.
Sulphate passes to the effluents by two main routes: from bleach plant filtrates and via rejected ESP dust from the recovery boiler. Bleach plant needs some sulfuric acid for pH control and either sulphur dioxide or sodium bisulphite (NaHSO3), which are needed for killing of the chlorine dioxide residues. On-site sources for bisulphite are today scarce, because there are no significant quantities of sulphur dioxide containing flue gases to be washed when lime kiln is heated with sulphur free on-site generated bio-based gas. Some sulphur is also captured into soda dregs and traces in lime, which are both rejected from the process at solid state. The reject rate of both routes tends to be pretty constant. Route to air as sulphur dioxide is also practically blocked today due to air pollution requirements. As the solid steams carry only little sulphur out from the process, the overall balance is that, practically all the sulphur entering the mill will be found in the effluents. So, the only solution to reduce the sulphate load in the effluent waters is to somehow reduce fresh sulfuric acid intake.
In chlorine dioxide production there are no realistic alternatives to sulfuric acid but in tall oil acidulation approximately half can be substituted with the aid of carbon dioxide. This was originally utilized to make sodium and sulphur balances match better together and to reduce the need for dumping of the recovery boiler ESP dust, which is mostly sodium sulphate. This means, that in larger scale, excess of sulphur entering into the process can be reduced only by on-site production of sulphuric acid by utilization of the sulphur, which already exist in the cooking chemical circle as a raw material.
Kraft process produces sulphur rich gases as a side stream, which can be converted into sulfuric acid. As a consequence of this, recovery boiler ESP ash excess is remarkably reduced. This fly ash is, however, the most important route to remove non-process elements (potassium and chloride) from the process cycle. Reduced allowable loss rate of ash can be replaced by recirculation and cleaning of the ash. This makes it possible to reject the non-process elements out from the process with much smaller amounts of useful sodium and sulphur. The overall cut on sulphate discharges is remarkable and at the same time the need for sodium make-up is reduced bringing pay-back for these additional processes. This reduce the salinity of the effluents in general. On-site production of sulphuric acid from process steams is convenient method to reduce sulphate discharges but there are practically no other technically available methods for further reduction of sulphur intake.
In freshwater ecosystems, salt discharges are undesirable, even though entering of large quantities of salt is accepted from salting of the streets and highways. Natural amounts are in scale of single milligrams per litre. How much is there sulphate in the effluents of a modern biorefinery? In the KCF case, effort has been put to minimize the pulping-based sulphate discharges and for 600,000 Adt/a production 38 t/d SO4 discharge level is achievable corresponding to 1 100 mg/l SO4 in litre pulping based effluent water. This causes 2 mg/l increase on the sulphate concentration in the recipient water. The total salt (Na, Cl, Mg, Fe, Al) discharges are estimated to mount up to 84 t/d corresponding to 2500 mg/l concentration in pulping based effluent waters yielding 4 mg/l increase in the recipient water at 250 m3/s average flow rate.
In potable water, sulphate concentrations up to 250 mg/l are allowed to meet the quality norms of tap water. Sea water at 3,5% salinity contains sulphate 2700 mg/l. Sulphate is not a strange ingredient in nature, though natural concentrations in Finnish lakes are low, less than 1 mg/l.
Vesa Mikkonen was born in 1966. He has always been interested in energy technology and natural sciences. Vesa has a MSc. degree in chemical engineering from Aalto-university, 1990.
 After finishing his studies, Vesa started working at the Äänekoski top modern pulp mill. He has worked a total of 18 years in the pulp industry in various positions developing processes and production efficiency at all kraft pulp mill departments and making plans and techno-economical calculations for entire new bio refinery concepts. Vesa’s strength is to put process into numbers to dig out its feasibility and applicability.
After finishing his studies, Vesa started working at the Äänekoski top modern pulp mill. He has worked a total of 18 years in the pulp industry in various positions developing processes and production efficiency at all kraft pulp mill departments and making plans and techno-economical calculations for entire new bio refinery concepts. Vesa’s strength is to put process into numbers to dig out its feasibility and applicability.
Vesa takes a special interest in the development of renewable energy, where he has made a reputation especially in the field of small-scale gasification. Batch and continuously operating biomass gasifiers, which he has developed, are used in road transportation, CHP power generation, boilers, kilns and even boats. Over a period of four years, he has worked with four different gasification technology companies.
Vesa has practical way of thinking and likes to do something with his hands. The mobile gasifiers he has built have been recognized for their design and craftsmanship. As an inventor, he holds a patent in the field of gasification technology. Currently Vesa is working as technical director at KaiCell Fibers and is responsible for EIA and permitting process.
Vesa is married to Ukrainian Vita and has two children. In addition to his Finnish native language, he is fluent in English and Russian. Vesa’s motto is: Whatever you do, do it properly! He has been involved with NC Partnering since 2013.
Source
NC Partnering, newsletter, 2019-03.
Supplier
KaiCell Fibers Ltd.
NC Partnering Ltd
US Energy Information Administration (EIA)
Share
Renewable Carbon News – Daily Newsletter
Subscribe to our daily email newsletter – the world's leading newsletter on renewable materials and chemicals










