“The Canadian Hemp Trade Alliance is very disappointed in the United Nations decision to continue restricting the global sales hemp-derived low-THC extracts,” said Ted Haney President and CEO of the Calgary based CHTA.
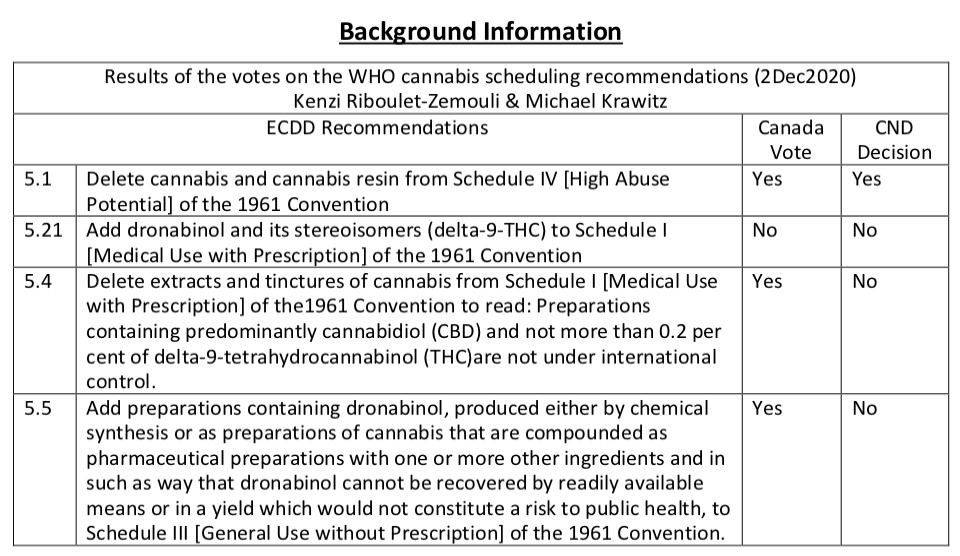
The United Nations Commission on Narcotic Drugs (CND), which met last December in Vienna, Austria, did not support the following five WHO Expert Committee on Drug Dependence (ECDD) recommendations to:
Delete dronabinol and its stereoisomers (delta-9-tetrahydrocannabinol) from the 1971 UN Convention on Psychotropic Substances (C71), Schedule II [Medical Use by Prescription], subject to the Commission’s adoption of the recommendation to add dronabinol and its stereoisomers (delta- 9- tetrahydrocannabinol) to Schedule I of the 1961 Single Convention on Narcotic Drugs (ECDD, 41st Report, Page 47);
Delete tetrahydrocannabinol (understood to refer to the six isomers currently listed in Schedule I [High Abuse Potential] of the 1971 Convention on Psychotropic Substances) from the 1971 UN Convention on Psychotropic Substances, subject to the Commission’s adoption of the recommendation to add tetrahydrocannabinol to Schedule I of the 1961 Single Convention on Narcotic Drugs (ECDD, 41st Report, Page 49);
Delete “extracts and tinctures of cannabis” from Schedule I [Medical Use by Prescription] of the 1961 UN Single Convention on Narcotic Drugs (C61) (ECDD, 41st Report, Page 53);
Add a footnote to Schedule I of the 1961 UN Single Convention on Narcotic Drugs to read “Preparations containing predominantly cannabidiol and not more than 0.2 per cent of delta-9- tetrahydrocannabinol are not under international control” (ECDD, 41st Report, Page 54).and to;
Add preparations containing delta-9-tetrahydrocannabinol (dronabinol), produced either by chemical synthesis or as a preparation of cannabis, that are compounded as pharmaceutical preparations with one or more other ingredients and in such a way that delta-9- tetrahydrocannabinol (dronabinol) cannot be recovered by readily available means or in a yield which would constitute a risk to public health, to Schedule III [General Use without Prescription] of the 1961 UN Single Convention on Narcotic Drugs (ECDD, 41st Report, Page 55).
“The CHTA believes that the United Nations made an error in not supporting the above C61 and C71 World Health Organization (WHO) Expert Committee recommendations that set out a THC threshold for “predominantly CBD preparations,” continued Haney. “By rejecting the changes, as recommended by the WHO’s own experts on drug dependence, the CND has shown its willingness to allow politics to intervene in what should be a science-based decision. The CND scheduling decision continues to impede the sale of safe and legal hemp-derived low-THC extracts in supermarkets, pharmacies, and health food stores; locations where Canadian consumers prefer to seek health and wellness advice and purchase natural health products. This remaining restriction makes it exceedingly difficult for the legal domestic and international hemp extract markets to develop – markets that would help Canadian and global farmers, processors, and consumers.”
Haney also confirmed that the CND correctly decided to remove hemp-derived low-THC extracts from the most restrictive C61 Schedule IV High Abuse Potential). This positive change allows for hemp and cannabis extracts to be sold globally as a medical ingredient by prescription and under the care of medical professionals. This is consistent with Canada’s decision to create a national medical cannabis market within the 2018 Cannabis Act and under the previous Cannabis for Medical Purposes Regulations (ACMPR).
Canada’s Paul Williams, the first secretary for his country’s [Canada] Permanent Mission to the United Nations, echoed his U.S. peer in acknowledging that Recommendation 5.5 was supported by scientific evidence, and that “CBD does not satisfy the criteria for international control under the drug conventions which are concerned with the risk of abuse and dependence.” Williams recalled several member states’ concern over the use of a footnote to clarify this situation. (Hemp Industry Daily, December 2, 2020).
It should be noted that Canada’s support for removing intergovernmental restrictions on the trade in hemp-derived low-THC extracts is consistent with the 2018 Cannabis Act, whereby cannabis extracts – with low and high levels of THC – are allowed for sale outside of the medical sector and within provincial, territorial, and indigenous regulated retail markets. Further, Canada’s vote in favour to remove international restrictions on the trade in hemp-derived low-THC extracts reflects the Government of Canada’s commitment to develop regulations using risk-based models that must reflect both regulatory objectives and economic outcomes of the regulated industries (Treasury Board of Canada Secretariat).
Canada’s support at the CND for allowing the General Use of hemp-derived low-THC extracts and preparations without medical prescription (ECDD Recommendation 5.5) is consistent with CHTA’s call for such products to be sold domestically outside of Cannabis Act controls. This will allow Canadian consumers to obtain health and wellbeing advice and purchase natural health products containing hemp-derived low-THC extracts at their preferred locations; supermarkets, pharmacies, and health food stores. Further, CHTA calls on Health Canada to allow Licenced Cannabis Processors (LPs) to sell hemp- derived low-THC extracts to Natural Health Product Site Licence Holders outside of Cannabis Act controls, allowing the natural health sector to create natural health products containing hemp-derived low-THC extracts outside of Cannabis Act controls, and to sell these products to supermarkets, pharmacies, health food stores, and directly to consumers. It is time for Health Canada to exempt domestic sales of hemp-derived low-THC extracts, and natural health products containing such extracts, from Cannabis Act controls.
Haney went on to explain that the CHTA was a major contributor to an international paper supporting the global liberalization of hemp-derived low-THC extracts, known as the ”The Common Position of the Industrial Hemp Sector on the Single Convention and the International Drug Control System.”
The paper was jointly issued by the following hemp associations: Asia-Pacific CBD Union; Australian Industrial Hemp Alliance; British Hemp Alliance; Canadian Hemp Trade Alliance; European Industrial Hemp Association; Hokkaido Hemp Association; Hemp Industries Association (USA); Latin American Industrial Hemp Alliance; Mongolian Hemp Association; National Hemp Association (USA); and New Zealand Hemp Industries Association. Special recognition goes to the European Industrial Hemp Association for their leadership on this project.
“One of the major roadblocks to growth in the hemp industry is that countries around the world regulate hemp in different ways,” explained Ted Haney. “Some treat hemp extracts as an agricultural product, while others treat them as a narcotic. The current patchwork of regulations makes selling hemp extracts internationally very difficult. The December 2020 decision by the CND has not fixed the situation.” Canada has an opportunity to provide global leadership in regulating hemp for OTC natural health products, just as it has for hemp foods; however, this opportunity is being squandered by a lack of regulatory agility by Health Canada.
Haney concluded by saying that the CHTA will continue to fight for its members, and with other countries, to encourage the Government of Canada and the CND to support the removal of intergovernmental, international, and domestic controls over the sales of hemp chaff/biomass (flowers, leaves, and branches) and hemp-derived low-THC extracts.
About the CHTA
CHTA is a national industry association that promotes Canadian hemp and hemp products globally. Established in 2003, the Alliance represents those involved in Canada’s hemp industry. Members include farmers, processors, manufacturers, researchers, entrepreneurs, and marketers.
The key functions of the Alliance are to disseminate information, promote the use of hemp products, develop standards, and coordinate research.
Source
Canadian Hemp Trade Alliance, press release, 2021-02-11.
Supplier
Australian Industrial Hemp Alliance
British Hemp Alliance
Canadian Hemp Trade Alliance CHTA
European Industrial Hemp Association (EIHA)
Government of Canada
Hemp Industries Association (HIA)
Hokkaido Hemp Association HIHA
Latin American Industrial Hemp Alliance LAIHA
National Hemp Association
New Zealand Hemp Industries Association
The Commission on Narcotic Drugs UNODC
World Health Organization (WHO)
Share
Renewable Carbon News – Daily Newsletter
Subscribe to our daily email newsletter – the world's leading newsletter on renewable materials and chemicals









