Catalytic Hydroesterification of Alkyl Lactates Provides Quantitative Yields
A new, sustainable method synthesizes acrylic acid and acrylate esters starting from alkyl lactates. The method reacts alkyl lactate with carbon monoxide and ethylene in presence of a palladium catalyst, resulting in catalytic hydroesterification of the alkyl lactates yields alkyl 2-(propionyloxy)propanoates. Pyrolysis of the alkyl 2-(propionyloxy)propanoates yields acrylate esters and propionic acid, and further hydrolysis of the acrylate esters yields acrylic acid. The synthetic method provides quantitative yields of the 2-(propionyloxy)propanoates, making it ideal for scale-up use in industry, and the catalytic species can be generated in situ in both in the neat alkyl lactate and in organic solvent from inexpensive and readily available starting materials.
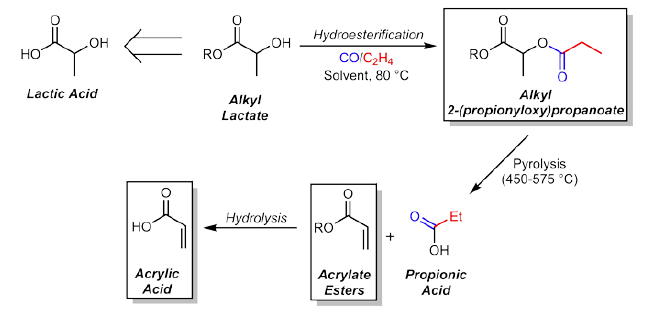
Biorenewable Starting Materials
Acrylic esters are currently derived directly from acrylic acid produced from byproducts of ethylene and gasoline production. Moving away from petroleum based feedstocks towards a biorenewable starting material is of key interest, and the technology presented here provides a viable route from bio-derived lactate esters to acrylic esters via a catalytic, two-step process. The synthesis can be done under neat conditions, avoiding the need for solvent, and takes place at just 80 degrees Celsius, a significant improvement over other methods that require temperatures higher than 250 Celsius.
BENEFITS AND FEATURES:
- Bio-renewable lactic acid used as starting material
- Hydroesterification using CO and ethylene in presence of palladium catalyst
- Simple catalytic, two-step process
- Uses inexpensive and readily available feedstocks
- Does not require solvents
- High yield; alkyl 2-propionyloxy propanoate intermediate yields are quantitative
- Conversion to acrylate and propionic acid via simple pryrolysis
- Relatively inexpensive reactants and low energy costs
APPLICATIONS:
- Bio-derived synthesis of acrylic acid and acrylate esters
- Bio-derived polyacrylic acid and polyacrylate polymers
- Bio-derived propionic acid for animal feed and food preservative
Phase of Development – Proof of Concept
Source
University of Minnesota, 2018-01-06.
Share
Renewable Carbon News – Daily Newsletter
Subscribe to our daily email newsletter – the world's leading newsletter on renewable materials and chemicals









